Abstract
Background. The incorporation of tyrosine kinase inhibitors (TKIs) in treatment schemes of Ph+ ALL has remarkably improved survival. In adult patients with Ph+ ALL, ponatinib in combination with chemotherapy showed a 3-year event-free survival rate of 69%, a 3-year overall survival (OS) of 83%, and a higher rate of response when compared with dasatinib plus chemotherapy. However, in unfit or elderly ALL patients, TKIs combined with chemotherapy are associations with higher toxicity. Therefore, we examined the efficacy and safety of steroids plus ponatinib alone for the treatment of elderly or unfit patients with Ph+ ALL in a multi-center Phase II prospective clinical Italian trial, GIMEMA LAL1811 (EudraCT number 2012-002761-35).
Methods. From March 2014 to December 2016, we enrolled 44 patients with untreated Ph+ ALL, ≥ 60 years or unfit (i.e. for intensive chemotherapy and stem cell transplantation). Two out of 44 patients were not elegible for the study.
Patients received oral administration of 45 mg/day of ponatinib for 8 consecutive courses of 6 weeks (w). Steroids were administered from day -14 to day 29 during course 1. Intrathecal therapy with methotrexate, cytarabine and dexametasone was performed every 28 days for central nervous system (CNS) disease prophylaxis. In patients with CNS disease at diagnosis, intrathecal therapy was administered twice a week until complete remission. Dose reduction of ponatinib was allowed for adverse events. Patient samples were obtained at diagnosis and at every course, BCR-ABL mutational analisys and BCR-ABL/ABL ratio by quantitative real time PCR was performed. Complete molecular response (CMR) was defined as BCR-ABL/ABL ratio below 0.01 or undetectable, and with a sensitivity of at least 30,000 molecules of ABL.
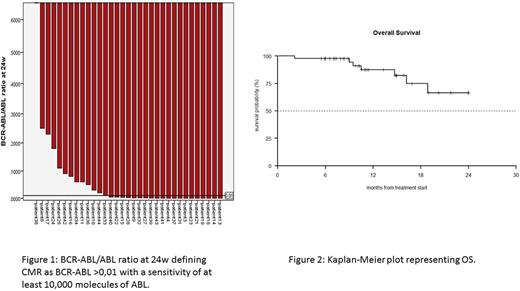
Results. Forty-two patients were eligible for the study. Median age was 68 years (range 27-85). Nine out of 42 patients were <60 years and were considered unfit. Twenty-six out of 42 patients had the p190 fusion transcript, 4/42 had p210, 12/42 had p190/p210. Steroid pretreatment was administered to 39; 14/39 patients had a reduction in circulating blasts of 75% or more before starting ponatinib. Primary endpoint (Complete hematological response (CHR) at 24w in 75% of patients) was prematurely reached. CHR was obtained in 40/42 patients (95,2%) after course 1 (6w). Thirty-eight out of 42 patients (90,5%) were in CHR after 8 courses (24w); 2 patients stopped treatment after 6w for disease relapse (1) and for excessive toxicity (1). Two patients dropped out after 12w for medical decision. A CMR was detected in 11/24 patients at 24w (45.8%; 14/38 patients not evaluable). Considering a CMR test sensitivity of at least 10,000 ABL molecules and testing peripheral blood whenever a bone marrow was not obtained, 20/33 patients (60.6% 5/38 patients not evaluable) were in CMR at 24w (figure 1). The median follow-up of the enrolled patients was 11.4 months (range 6-34.5). Overall survival (OS) at 6 months and 1 year was 97.6% (C.I 95%: 93.1%-100.0%) and 87.5% (C.I. 95%: 76.5%-99,9%) respectively (figure2).
At week 24, 15/42 patients still received 45 mg of ponatinib daily, only 4/42 patients permanently withdrew study drug. During the study, 75 adverse events (AE) were reported; 36 of the 75 AEs were considered related to ponatinib. Twenty-six of the 75 AEs were considered serious (SAE); 13/26 SAEs were considered related to ponatinib. A death was suspected to be related to ponatinib.
We performed BCR-ABL mutational analysis in 22 patients at diagnosis, and 15 patients at 24w. T315L (abundance 100%) was detected in a patient relapsed during ponatinib therapy. We could not identify the emergency of other mutations.
Conclusions. Ponatinib and steroid show a high efficacy in newly diagnosed unfit/elderly Ph+ ALL patients. Toxicities were manageable and cardiovascular AEs were limited. In the small cohort of patients relapsed in the study, relapse mechanisms were unclear; only one patient had evidence of mutations that caused resistance to ponatinib. The fast and deep reduction of the disease burden in the majority of patients, the ability of ponatinib to prevent the emergence of clones harboring BCR-ABL mutations, and the synthetic lethality with steroids on the BCR-ABL, FLT3, HCK, CDK6, MCL1 pathway could explain the therapeutic effectiveness.
Acknowledgments. GIMEMA, ELN, AIL, AIRC, Regione-Università 2010-12, FP7 NGS-PTL, HARMONY, Fondazione del Monte BO e RA.
Soverini: Bristol-Myers Squibb: Consultancy; Incyte Biosciences: Consultancy; Novartis: Consultancy. Bocchia: Novartis: Other: Travel grant; Celgene: Other: Travel grant; Roche: Other: Travel grant; Jansen: Other: Travel grant. Cuneo: Abbvie: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board; Gilead: Honoraria, Other: Advisory Board; Roche: Honoraria, Other: Advisory Board. Bonifacio: Pfizer: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees. Falini: Roche: Research Funding. Galieni: Takeda: Other: Advisory Board; Abbvie: Other: Advisory Board. Foà: Sandoz: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; janssen: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Baccarani: Pfizer: Honoraria, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Speakers Bureau; Incyte ARIAD: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal